What Is the Second Law of Thermodynamics Quizlet
The second law of thermodynamics states that entropy which is often thought of as simple disorder will always increase within a closed system. 2nd Law of Thermodynamics.

Introduction To The Second Law Of Thermodynamics Heat Engines And Their Efficiency Physics
The second law of thermodynamics states that the total entropy can only increase over time for an isolated system meaning a system which neither energy nor matter can enter or leave.

. The Second Law also states that there is a natural tendency of any isolated system to degenerate into a more disordered state. Second Law of Thermodynamics DRAFT. The Second Law of Thermodynamics states that when energy is transferred there will be less energy available at the end of the transfer process than at the beginning.
The second law of thermodynamics states that entropy of a system tends to _____. Isolated systems spontaneously evolve towards thermodynamic equilibrium the state with maximum entropy. The law of entropy which states that every transfer of energy or change increases the amount of entropy in the cosmos.
Isolated systems spontaneously evolve towards thermodynamic equilibrium the state with maximum entropy. The First Law because all energy on Earth originates from the Sun. What does the 1st law of thermodynamics state quizlet.
The second law of thermodynamics means hot things always cool unless you do something to stop them. You might be interested. Energy can only be transferred or changed from one form to another.
Due to entropy which is the measure of disorder in a closed system all of the available energy will not be useful to the organism. It expresses a fundamental and simple truth about the universe. Isolated systems spontaneously evolve towards thermal equilibriumthe state of maximum entropy of the system.
In a developed system it is called an isolated system and the state of maximum entropy is reached without any process intervention. How do you prove the first law of thermodynamics. The second fundamental law of thermodynamics states that when a hot object has been placed near a cold one its heat flows from the hot to the cold.
A heated object can still be a part of the energy supply if it passes into the warmer one via the hot object. If a certain series of elements become en vogue through t thermoregulatorys the entropy of that series can increase every time. The second law of thermodynamics states that the total entropy of an isolated system can never decrease over time and is constant if and only if all processes are reversible.
The Second Law because the Sun provides energy to the Earth thus making. Mathematically the second law of thermodynamics is represented as. That disorder characterised as a quantity known as entropy always increases.
It states that as energy is transferred or transformed more and more of it is wasted. The Second Law of Thermodynamics is about the quality of energy. Questions in other subjects.
Second Law of Thermodynamics DRAFT. The _____ Law of Thermodynamics says that heat always flows from an object with a higher temperature to an object of lower temperature naturally. The second law of thermodynamics says that the entropy of any isolated system always increases.
The second law of thermodynamics is as follows. Ultimately this is one of the key elements dictating an arrow of time in the Universe. What is the second law of thermodynamics quizlet.
Preview this quiz on Quizizz. At the very least ordered types of energy are turned to heat to some extent. ΔS univ 0.
Second Law of Thermodynamics Equation. The entropy of the universe the ultimate isolated system only increases and never decreases. How does the first law of thermodynamics apply to environmental science.
Where ΔS univ is the change in the entropy of the universe. How is active transport possible since it contradicts the second law of thermodynamics. The First Law because no energy escapes from Earth it stays on the planet indefinitely.
The second law of thermodynamics states that the total entropy of an isolated system can never decrease over time and is constant if and only if all processes are reversible. What is the law of octaves. Entropy is a measure of the randomness of the system or it is the measure of energy or chaos within an isolated system.
Gcf of 8 and 52 for. The second law of thermodynamics states that quizlet. What Is The Second Law Of Thermodynamics In Chemistry.
The first law of thermodynamics also known as Law of Conservation of Energy states that energy can neither be created nor destroyed. The second law of thermodynamics states that entropy of a system tends to _____. Not one could ever spontaneously produce heat from that hot to the cold from the cold.

Second Law Of Thermodynamics And Entropy Video Khan Academy

How Does The Second Law Of Thermodynamics Relate To The Direction Of Heat Flow Lisbdnet Com
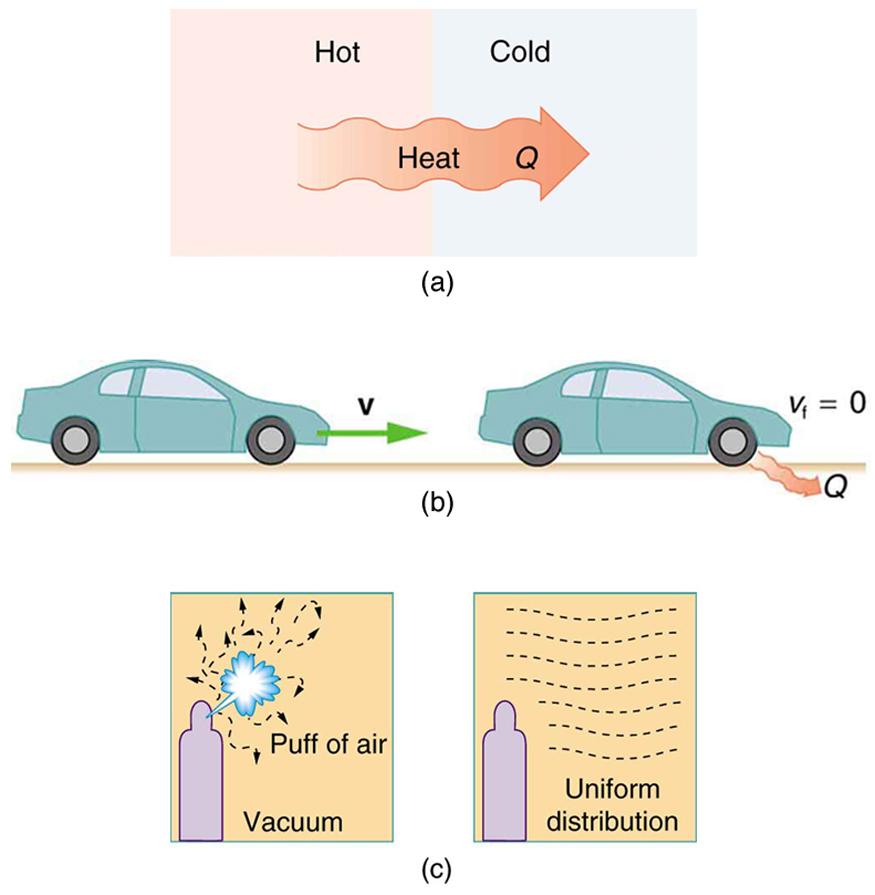
Introduction To The Second Law Of Thermodynamics Heat Engines And Their Efficiency College Physics

Physics Chapter 10 Thermodynamics Diagram Quizlet

3 Second Law Of Thermodynamics And Entropy Flashcards Quizlet

Science Using Heat Flashcards Quizlet

Second Law Of Thermodynamics Flashcards Quizlet

4 Laws Of Thermodynamics Science Guide Thermodynamics Physics Physics Formulas

Second Law Of Thermodynamics Second Law Of Thermodynamics Physics Notes Thermodynamics

Thermodynamics Vocab Flashcards Quizlet

Solved Which Best Describes The Second Law Of Chegg Com

Difference Between First And Second Law Of Thermodynamics Differbetween

Does Evolution Violates Second Law Of Thermodynamics

Newton S Laws And Friction Pictures Planets In The Sky Upanishads Gravitation

My Praxis Chm Set 5245 Flashcards Practice Test Quizlet

Second Law Of Thermodynamics Isolated System Poster Zazzle Com In 2022 Second Law Of Thermodynamics Thermodynamics Physics


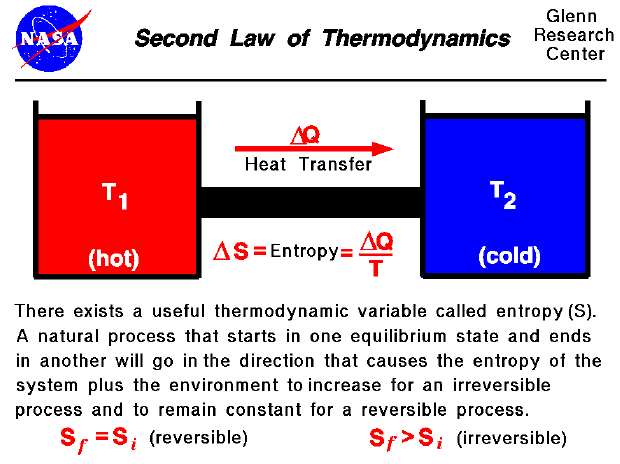
Comments
Post a Comment